Dengue is a mosquito-transmitted virus and the leading cause of arthropod-borne viral disease in the world. It is also known as breakbone fever due to the severity of muscle spasms and joint pain, dandy fever, or seven-day fever because of the usual duration of symptoms. Although most cases are asymptomatic, severe illness and death may occur. There is currently no specific medicine for dengue treatment, and prevention majorly relies on vector control.
About half of the world's population is now at risk of dengue with an estimated 100-400 million infections occurring each year. Dengue is prevalent in tropical and subtropical climates worldwide, mostly in urban and suburban areas.
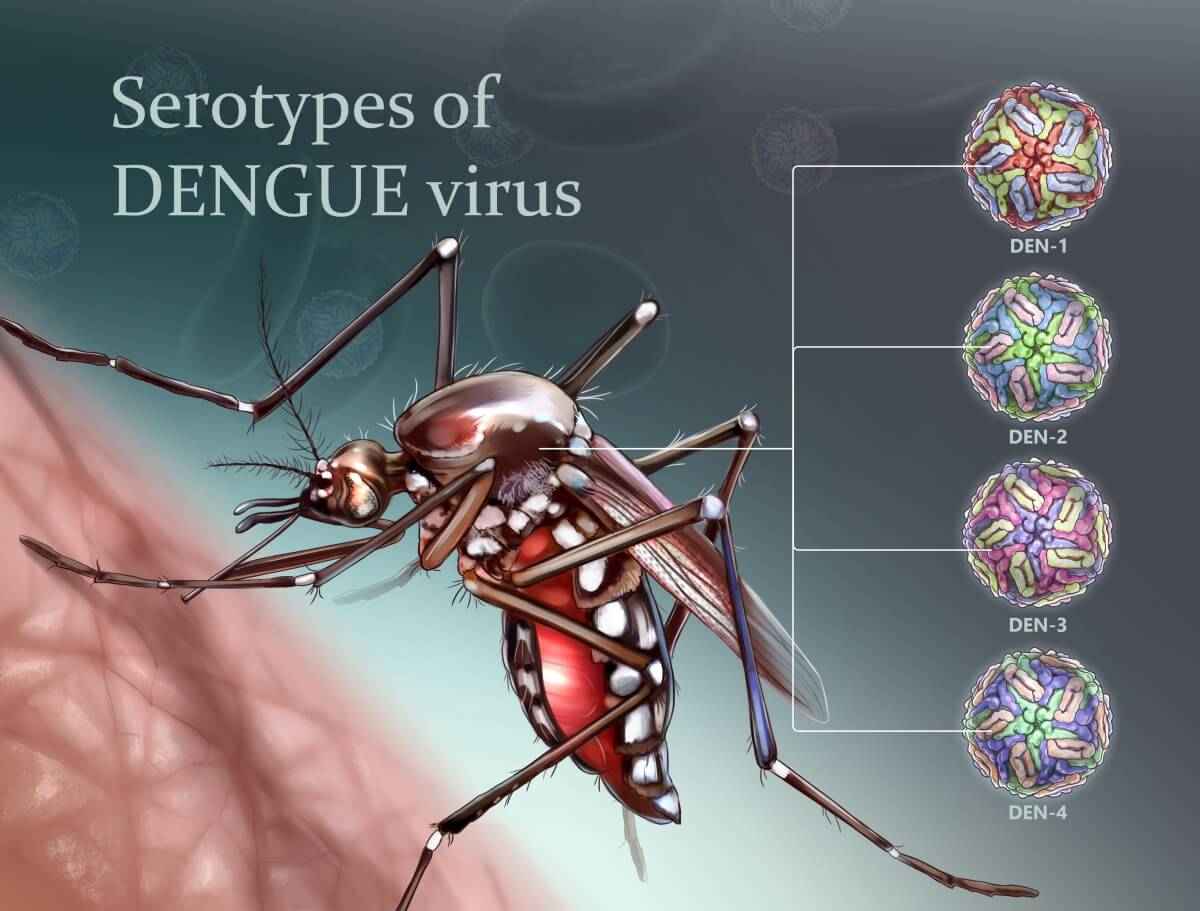
There are four serotypes of dengue virus (DENV1–4) belonging to the Flaviviridae family, Flavivirus genus. DENV is transmitted by Aedes spp. mosquitoes, primarily Aedes aegypti. Dengue disease is particularly prevalent in tropical and subtropical areas of the world, where Ae. aegypti has managed to occupy an ecological niche alongside humans.

All four serotypes can cause dengue fever, dengue hemorrhagic fever, and dengue shock syndrome. Dengue hemorrhagic fever and dengue shock syndrome are the more severe sequelae and are most commonly observed in infected children and adolescents under the age of fifteen. DENV infection promotes the formation of high titers of neutralizing antibodies, which are considered an essential component of the protective immune response. Protection against infection with one serotype is considered long-term after infection with one serotype, while cross-protection against infection with another serotype may last for about two years.
Furthermore, non-neutralizing antibodies can form complexes with DENV particles and facilitate virus infection to phagocytic cells via Fc receptors, resulting in enhanced infection and severe complications. This phenomenon is called antibody-dependent enhancement. So, DENV vaccines must protect against infection from all four serotypes.

Development began in the 1920s and involved attenuating DENV in the blood using ox bile or grinding DENV-infected Ae. aegypti mosquitoes in saline and chemically pure phenol and formalin. However, the development was hindered by the need to create immunity against all four dengue serotypes.
Today, three vaccines (Dengvaxia, QDENGA, and TV-003/005) have shown promising results. They all use recombinant live attenuated viruses.
CYD-TDV developed by Sanofi Pasteur, TAK-003 developed by Takeda, and TV003/TV005 developed by the National Institute of Allergy and Infectious Diseases (NIAID) use recombinant live attenuated viruses.
Dengvaxia (CYD-TDV) vaccine is made by Sanofi Pasteur and became commercially available in 2016 in 11 endemic countries. In 2019, it was approved for medical use in the United States. It is on the World Health Organization's List of Essential Medicines.
QDENGA (TAK-003) is a dengue vaccine made by Takeda. Takeda's QDENGA is approved in endemic countries and the European Union.

Both Dengvaxia and QDENGA vaccines are tetravalent live-attenuated viruses. However, Dendvaxia's protection efficacy was significantly distinct against different DENV serotypes: highest against DENV3 and DENV4 and lowest against DENV2. Similarly, in TAK-003, protection efficacy was highest against DENV2 and lowest against DENV4.
The National Institute of Allergy and Infectious Diseases is developing the dengue live attenuated tetravalent vaccines TV003 and TV005 undergoing phase 2 and 3 clinical trials.
Other 15 vaccines are under development, introducing novel immunization strategies to the traditional dengue vaccine scenario. Clinical trials for TDENV-PIV, the DNA vaccine TVDVVAX, and the subunit vaccine DEN80E35 showed very encouraging results, although these novel techniques may be less immunogenic than live-attenuated vaccines. A heterologous prime/bust approach, combining different vaccine platforms to overcome the shortcomings of one vaccine category and achieve a more balanced and robust protective immunity, appears most promising.
Dengue remains a major health concern today. Its prevention in populations living in endemic regions is essential, but with many dengue vaccine candidates in various stages of clinical trials, we will see more licensed dengue vaccines in the future.